[ƴ��]��jinshu ranliao
[����]��metal fuels
һ���Ը�ȼ����ֵ�Ľ���Ϊ��Ҫ��ֵ�ȼ�ϡ����Ȼ�ѧ�Ĺ۵㿴����Ԫ�����ڱ������ϽDz��֣�ԭ����С�Ľ�������ʱ�������Ի�øߵ�ȼ���ȡ����ǵ�λ������ȼ�ϼ�����������ȼ���Ⱥͳ���ȼ�ϣ����͡��⣩�ıȽϼ�ͼ���ɴ˿����롢ﮡ�������þ���ѡ���Ƚ����ĵ�λȼ���ȴ������ͺ����ȼ���ȡ�
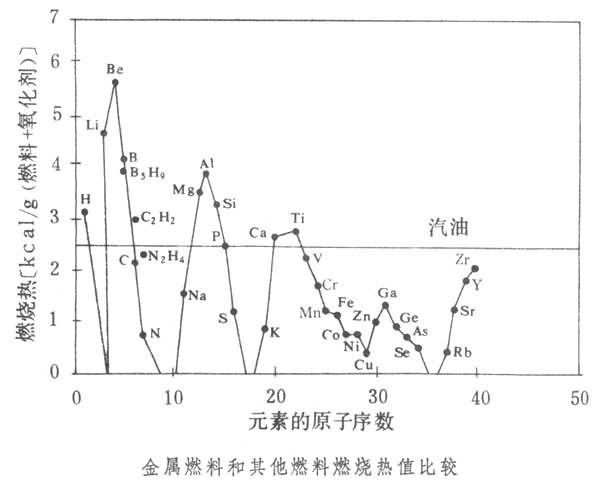
������������������ʹ��ȼ���ȸߵ�����ȼ����������Һ������ȼ������������Ϊ 0.1��50��������ĩ������������(��LiH��BeH2��B2H6����gH2��AlH3��)������������(��LiNH2)������̼�⻯����LiC2H5��Be(CH3)2��B(CH3)3��Al(C2H5)3�ȣ����Ի�øߵ�������
���ڽ���ȼ�ϵĻ����¶ȸߣ�ȼ�ղ�������ķ���ϵ���ߣ����γɷdz������Ļ��档����㷺ʹ��þ���ѻ����ȼ�յ�������������������������ơ�
ըҩ�����ӽ�������߱��������ƽ��������� TNTըҩ������15�������ۣ������������20���������������30�������������������ҩ�ij�������ԡ���������������и��մɺ�����������Ȼ����ȼ���������ԣ����۸�ߣ����ڵ�𣬲��ױ����ȶ��Ļ��棬Ӧ���ܵ����ơ�