������ˮ������������Ҫ��������ѧ��������֮һ����һ����ͨ�������Ⱥ�ʹС���������۳ɴ������Ŀǰ����ѧ�����Ŀɽ��̶ܳ�����С������Ҫ����Ϊ�뻯ѧ�Լ������йط��ð����磺�����������������ж��������ѧҩ���ȣ������������̿�ͨ����ѧ�͵�ѧ;������������������á�
1�������������ۻ���
������һ�����ӵĹ��̣��ڵ糡�������½����缫�����������ڽ���ˮ��ʱ��������������ѧ�������ӵIJ������γ�����������������ĽΣ�
��1���ڵ糡�������£��������������γɡ�������������������������������
��2��ˮ�������Ŀ�����������Ⱦ������������������ʧȥ�ȶ�����
��3�����Ⱥ����Ⱦ�������������֮�����ײ����ϳ����ۿɼ��Ĵ����塣
���ڵ����������е�ⷴӦ�IJ���ֻ�����ӣ�����ҪͶ���κ���������ԭ�����Ի�������������ٲ�����Ⱦ������Ϊ��һ�ֻ����Ѻ�ˮ���������������������кܶ���ŵ㣬�磺
��1���豸��ռ������٣��豸ά����
��2�������������в���Ҫ�����κλ�ѧҩ�����������������٣�������ĺ�ˮ�ʵͣ����ڴ�����
��3��������ֻ��Ҫ�ı�糡����ӵ�ѹ���ܿ������������ĸı䣬������ʵ���Զ������ƣ�
���������г��õĵ缫����Ϊ��������������������֮��ͨ��ֱ�����������ĵ缫��Ӧ���£�
������
Al��3e��Al3e+ �������������������� (1)
�ڼ���������
Al3e++3OH����Al(OH)3 ���������������� (2)
������������
Al3e++3H2O��Al(OH)3+3H+ ��������������3��
������
Fe��2e��Fe2e+ ������������������ (4)
�ڼ���������
Fe2e++2OH����Fe(OH)2 (5)
������������
4Fe2e++O2+2H2O��4Fe3e++4OH�� ��6��
���⣬ˮ�ĵ��������ų�
2H2O��4e��O2+4H+ ��7��
�������������·�Ӧ
2H2O+2e��H2+2OH�� ��8��
���������ڴ��������о��ж���ԣ����˵���������֮����绯ѧ�����ͻ�ԭ�������������á���������ȥ��ˮ����Ⱦ����̼�ͼ1��
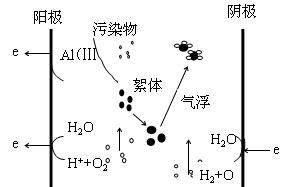 |
ͼ1 ������ȥ����Ⱦ�����
2����������Ӧ���е缫��Ϸ�ʽ
�ڵ��������У����յ缫������ĵ缫���Է֣����������ɷ�Ϊ����ʽ��˫��ʽ�����ʽ���࣬��ͼ2�����ڵ���ʽ�������������Ƹߵͽ������������Ǵ�ijһ�����������ڵ����������������ƹ����鼫����������������ÿ�鼫����ֳ�һ�ֵ��������ڵĵ缫����Ϊ��ͬ�ĵ��ԣ�����������������ڵ�����й©���⣻˫��ʽ�����ʽ�������������ͬ�����ֵ��������ƹ����鼫�壬�ӿ�����Դ������һЩ����ֱ��������Դ������һЩ���壬�������Դ���������ļ����⣬ÿ�鼫����ֳ���ͬ�ĵ��ԣ�˫��ʽ�����ʽ�������ŵ���й©������
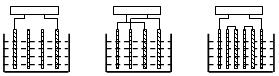 |
��a�� (b) (c)
ͼ2 ���������缫���ӷ�ʽ
(a) ����ʽ (b) ˫��ʽ (c) ���ʽ
3����ˮ�����е�Ӧ��
������������Ŀǰ�ܶ��ˮ��������Ӧ�ã��ڸ�Ũ�ȡ����������⡢Ҫ��߰���ȥ���ʵķ�ˮ�Ĵ����������ƣ���Ŀǰ���ղ�������ķ�ˮ�������������������ںܵ͵�Ͷ�������£�ʵ��ԭ�з�ˮ�������յij�ˮ��꣬�ɽ�ԭ������Ҫ�ĺķѶη������ã���ͼ3��
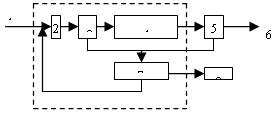 |
ͼ3 ��������ȡ�������ˮ�����е�����
1889�꣬Ӣ�������������缫��������������������ˮ��20����60������ڣ�����������ҵ��Ѹ�ٷ�չ���绯ѧ����ʼ�������ǵ�ע�⡣�������������������ѹ㷺��Ӧ���ڸ���ˮ�������С�Ŀǰ�������������ѳɹ���Ӧ���ڽ����Ļ��ա������л�Ⱦ�ϡ������������Ӹ�����ҵ��ˮ�������ˮ��ȥ����֬�Լ��������ַ�ˮ�Ĵ�����
Chen��[1]������Ϊ�缫���ϣ����õ���������������Ũ����֬�IJ�����ˮ������ʱ��С��4.5min����֬��COD��SS��ȥ���ʷֱ�Ϊ99%��88%��98%��ÿȥ��1kgCOD��������0.20-0.37kg��������Ϊ0.5kWh/(m3��ˮ)��������ij�ˮ�����ŷ�Ҫ����Ч����Գ��й���ˮ������ʩ�ĸ��ɡ�
��֯ӡȾҵ�ŷŵķ�ˮ�к��д������������オ���Ⱦ�Ϸ��Ӻͺܸߵ�COD�������������ŷŵ�ˮ���л�������Ⱦ���������õ�����������Ч��ȥ����ˮ�е�ɫ���Լ�����COD��Bayramoglu[2] �ȷֱ�������������Ϊ�缫�����˵�����������֯��ˮ���о�������Һ������ʱ���������缫ȥ��COD���ǶȵĴ���Ч��Ҫ�������缫��������ҺΪ���Ի�������ʱ���������缫����Ч��Ҫ�����缫�á��ڵ����������У���Һ�ĵ絼�ʽϸ������еķ��ýϵͣ��ڴﵽ��ͬ��ȥ��COD ���Ƕȵ�ȥ��Ч�ʣ��������缫�����缫��Ҫ���͵ĵ����ܶȡ�Alinsafi[3]�����õ�����������������Ⱦ��ӡȾ��ˮ���о���pH�������ܶȺͷ�Ӧʱ������ض�ȥ��ɫ�Ⱥ�COD��Ӱ�죬��ѷ�Ӧ105min�������ܶ�12mA/cm2��ɫ�ȵ�ȥ���ʴﵽ90-95%��COD��ȥ������30-36%֮�䡣
�����������ڴ���ijЩ��ҵ�����������ŷŵĺ�����Һ�����ȥ��Ч����Ҫȡ���ڵ����ܶȡ���ʼ��Һ�����Ũ���Լ�����ʱ�䣬�ڵ����ܶ�Ϊ20 mA/cm2������20-30min�����ȥ����ԼΪ90%����ʼŨ��Ϊ2.5g/L�����ܺ�Ϊ2.5-4.5kWh/m3��ˮ������Һ������CaCl2������ߵ絼�ʣ��������������ġ�
��ʯ�͡���Ȼ��������̽�����У��ۻ��ཬ��ϵ�������꾮��ˮ�к��д����Ķ���������л���,��COD��ɫ�ȸߡ��ȸߡ�������ߡ���������,����������ֱ���ŷţ����Ի��������Ⱦ�������ˮ���ó����������մ�������������ˮ����ŷţ��䴦���ڹ��ڻ�û�еõ����ƽ�������ij�[4]�Ȳ��õ����������Ծۻ��ཬ��ϵ�꾮��ˮ���д�������ˮ��75min������COD����1511mg/L�½���86.3mg/L��ɫ�ȿɴ�1500������27��������Ҫˮ��ָ�������GB8978-1996һ���ŷű�Ҫ��
���õ�����������ֽ��ˮ���о�����������ڵ�ѹΪ12V������Ϊ77.13mA������ʹ���������缫����2min�ڶ�����Чȥ����NO 3-�����������ֽ��ˮ�е���Ⱦ��������ӵ�����������ʱ�䣬��COD��BOD��ȥ���ʵ�Ӱ�첻��
��ơ�ұ��ȹ�ҵ����ͨ���ŷź�Cr 6+��Һ��������Ի���������Σ�������������ŷŶ����ϸ��Ҫ�����ҹ�Ҫ���ܸ����ŷű�Ϊ0.5mg/L��GB8978-1996������ͳ�����ķ����г��������������オ��ȣ�Gao[5]�ȶ����õ�����������ˮ�еĸ���Cr 6+������������Fe 2+��Ӧ������ԭΪCr 3+��������������OH-���ɳ�����ȥ�����ܸ�Ũ��Ϊ3.0g/L����Һ����������������Ũ��С��0.5mg/L�������������Ϊ������2.5F/m3��ˮ��pH5-8����������С��1kWh/m3��ˮ����ˮ�и���ȥ���ʳ���99����
����ͳ��ŷŵ����ɫ��ˮ�к��д����ķӣ��ŷŵ�ˮ���л�����ũҵ���֮ǰ������д�����Adhoum[6]�����õ���������������ͳ���ˮ������������25min��ˮ��COD����Ԫ���Լ�ɫ�ȵ�ȥ���ʷֱ�ﵽ76����91����95�������缫��������Ϊ2.11kg/m3��ˮ�����õ������������ϴ�������ͷ�ˮ��ȡ�ø��õĴ���Ч����
���Ƕ�ˮ��������Ϊ��ˮ�ʾ�����������������֮һ����������������������б�ܳ������������Ϲ��ˣ�����϶���������������¹��գ��ɽ��Ƕ�Ϊ10000�ȵ�ԭˮ��42min��һ�ξ���Ϊ�Ƕ�С��3�ȵ�����ˮ��ͨ���Կ�����������������о����֣��ڵ����������У��͵���ʱ������ͨ��������ȥ�����ߵ���ʱ������ͨ��������ȥ����
�����ۿ�ͬʱ��ȥˮ���л��ϸ�����ж��ؽ�����������������Ƕȣ���һ�ֺ���ǰ;�ĸ�ˮ����������
����ˮ�е�����Ҫ��As����As(��)������̬���ڣ�������߶�������Ӱ��������Ľ�����Kumar[7]���о����������õ�������ȥ��ˮAs����As(��)��Ч������� �ڵ�����������As�������ȱ�������As(��)��Ȼ��ͨ�������Լ����������������γ���λ��������ȥ�������ø÷������Ը��ߵ�As����Ч��Ҫ���ڴ�ͳ��ʹ��FeCl3��������
�ҹ�����ˮ�������涨��ˮ��������������0.3mg/l����ʹ�õ����ѹ��ʽ������SS���Ϲ��˳�����װ���У�ԭˮ�е�Fe 2+����Al��OH��3�������پ���SS���Ϲ��˺�ȥ������ԭˮ�е�������С��30mg/Lʱ���������ˮ���ܴﵽ��������ˮ��������
�������缫������Ⱦ�ĺ�ˮʱ��ϸ�����Ƕȵ�ȥ��Ч����ʮ����������������ܶȿ����COD��ϸ����ȥ���������ʣ����ﵽ��ͬȥ���ʵ��ܺĽ�����
4������
��������һ��ˮ�����;��������������о��������е����û�������Բ�ͬ�ķ�ˮѡ��ǡ���Ĵ������գ�ͨ���Ľ���Դ�������о����͵缫���ϼ������ṹ���Խ�һ����ߵ��������������Ĵ���Ч�ʺͽ����ܺ��ǵ�ǰ�ü����ķ�չ�������ŵ�����ҵ�ķ�չ�Լ��Ե绯ѧ���о�Խ��Խ���룬��������ˮ���������Ź㷺��Ӧ��ǰ����
�����
[1]��Chen Guohua, Chen Xueming, Yue P. L.. Electrocoagulation and electroflotation of restaurant wastewater. Journal of Environmental Engineering, 2000(a), 126(9): 858-863.
[2]��M. Bayramoglu, M. Kobya, O. T. Can, et al. Operating cost analysis of electrocoagulation of textile wastewater [J]. Separation and Purification Technology, 2004, 37(2): 117-125.
[3]��A. Alinsafi, M. Khemis, M. N. Pons, et al. Electro-coagulation of reactive dyes and textile wastewater [J]. Chemical Engineering and Processing, 2005, 44(4):461-470.
[4]�����ij�,Ҷ��,������,��.�������������ۻ��ཬ��ϵ�꾮��ˮ[J].��������,2004,,24(����):196-198.
[5]��Gao P., Chen, X. M., Shen, F. , et al. Removal of chromium(VI) from wastewater by combined electrocoagulation-electroflotation without a filter [J]. Separation and Purification Technology, 2005, 43 (2): 117-123.
[6]��Adhoum N., Monser L.. Decolourization and removal of phenolic compounds from wastewater by electrocoagulation [J]. Chemical Engineering and Processing, 2004, 43(10): 1281-1287.
[7]��P. R. Kumar, S. Chaudhari, K. Khilar, et al. Removal of arsenic from water by electrocoagulation [J]. Chemosphere, 2004, 55(9):1245-1252.














