1 ����
1.1 ����ˮ��
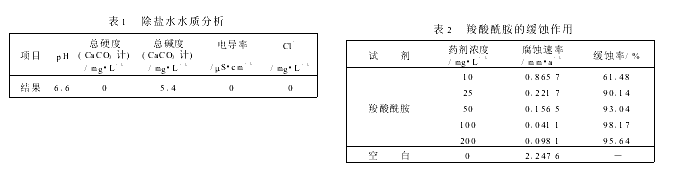
����ˮˮ�ʼ���1��
1.2 ����
RCC������ת��Ƭ�ǡ�
1.3 ���鷽��
������ҵ��HG/T2159��91��ʧ�����鷨�ⸯʴ�ʡ�
1.4 ��������
80�棬2.0L/����72h����Ƭ������0.315mm/s����Ũ����
1.5 ��Ƭ����
20#̼�֣�40mm��13mm��2.0mm�������������ṩ��
1.6 ��ѧ�Լ�
������(Na2MoO4.2H2O)��ҵ��������98%���������������ƣ���ҵ��������Լ80%��
2 ���������
2.1 ���������ĺϳ�
�����������л��ᡢ���ᡢ�л������ʵ��Ĵ������¶��ºϳɶ��ã��仯ѧ��Ӧʽ���£�
2RCOOH+H2N-(CH2CH2)n-NH2Cat
RCONH-(CH2CH2)n-NHCOR+2H2O
��̼ͬ�����л��ᡢ�л����Բ���Ļ�ʴ�����нϴ��Ӱ�죬һ����ԣ�̼��Խ�������Ļ�ʴ����Ч��Խ�ã���ˮ����Խ����ǵ���Ϊˮ��Ӧ�õ�ҩ������ˮ���Ժ����Ⱦ�����������ѡ���л�����л���ʱ�����˲��ﻺʴ���ܺ�ˮ���Ե�����ͳһ��
2.2 ���������Ļ�ʴ����
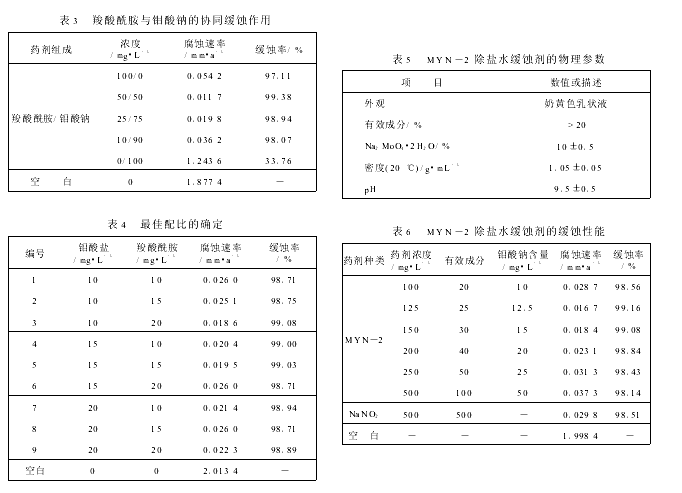
����ˮ�в�ͬŨ�ȵ�����������20#̼�����ü���2��
�ɱ�2��֪���ڳ���ˮ�е���ʹ�������������нϺõĻ�ʴЧ����Ũ����100mg/L����Ϊ��ѡ����ںϳɵ���������Ϊ������������Լ����н�ǿ����ϴ�����������ã���Ũ��̫��ʱ�Ի�ʴ�����
2 3 ���������������ε�ЭͬЧӦ
Ϊ�˽���ҩ����ʹ��Ũ�ȣ����������������������߸���ʹ�ã�����������3��
��3�����������������������������ԵĻ�ʴЭͬЧӦ�����������������Ƹ���ʹ�ÿ��Դ�����ߵ�ʹ��Ũ�ȡ�
2.4 ������������������������
Ϊ��һ������ҩ��Ũ�ȣ����ʴЧ�������ǽ����˲�ͬ��ȵ����飬�������4��
��4��֪��������10mg/L����������10mg/Lʱ���м��õĻ�ʴЧ������ʴ�ʴ�98%���ϡ�
2.5 ����ҩ��������
�������ơ���������=10��10�ı���������1%���ҵı�����Լ�����������ˮϡ�ͣ���һ���ɫ��״Һ����������ΪMYN��2����ˮ��ʴ�����䲿���������ܼ���5��
MYN��2�ڳ���ˮ�еĻ�ʴ���ܼ���6��
2.6 Ӱ��MYN��2��ʴЧ��������
Ӱ��MYN��2��ʴЧ����������Ҫ��pH���¶ȡ�Cl-Ũ�ȵȡ�����ˮ��pHֵͨ����NH3���ڣ�Cl-Ũ����NaCl�ļ��������ƣ���������ͬ�������顣������������pHֵ���¶ȶ�MYN��2�Ļ�ʴ���ܻ�����Ӱ�죬Cl-�Ĵ��ڴ�������MYN��2�Ļ�ʴ���ܣ�����Cl-Ũ�ȵ����ߣ�MYN��2��ɥʧ��ʴ���á�Ҳ����MYN��2���ʺ��ں�Cl-������ˮ��ʹ�ã�ֻ���ڳ���ˮ(��������ȴˮ)������ʴ���á�
3 ����
���Ƶ������������������ڳ���ˮ�ж�̼�ִ������ԵĻ�ʴЭͬЧӦ���������ơ���������Ϊ���帴�϶��ɵij���ˮ��ʴ��MYN��2�����������͡���ʴ���ܺá���ӦpHֵ���¶ȷ�Χ�������Ƶ��Դ���ŵ㡣��Cl-�����м����Ӱ�죬�����ں�Cl-��ˮ����ʹ�á�














